About one hundred years ago, Chinese hamsters (Cricetulus griseus) had left their hometown in the deserts of China and “entered” the laboratory for molecular typing of Streptococcus pneumoniae. In the late 1950s, Dr Theodore Thomas Puck from the University of Colorado’s Department of Medicine had isolated and studied the ovarian tissue from a female Chinese hamster which the original CHO-K1 cell line was derived. Since then, Chinese hamster ovary (CHO) cells sparked many laboratory studies, diverged into different adherent and suspension cell lines, such as CHO‐K1, CHO‐S, and CHO DG44. The characteristics of non-immunogenic glycosylation, fast growth, and ease of genetic manipulation along with publicly accessible CHO genome sequence have ultimately led to their selection as industrial host expression system of choice for today’s $30bn biomanufacturing industry, covering nearly 70 % of all recombinant biotherapeutics production.
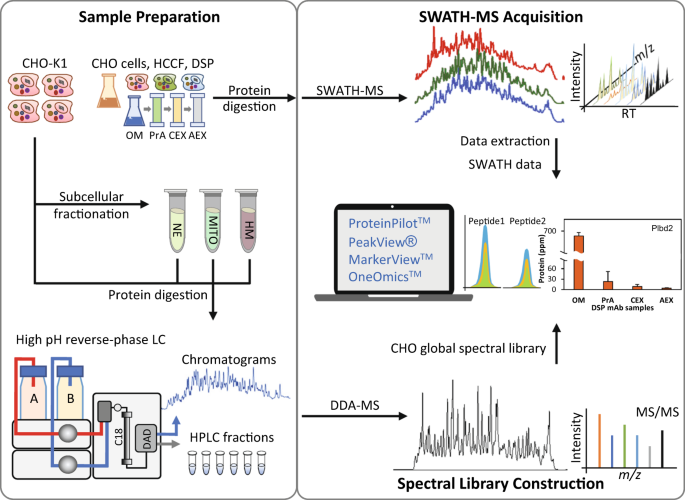
In a collaborative project led by Dr. Xuezhi Bi (Bioprocessing Technology Institute, A*STAR, Singapore) and Dr Stephen Tate (SCIEX, Canada), the team proposed to study the CHO cell lines and downstream processing (DSP) mAb samples using the state-of-the-art SWATH-MS (sequential window acquisition of all theoretical fragment-ion spectra) technique. This data-independent acquisition (DIA)-MS method, coupled with peptide-centric data extraction strategy, provides the possibility to overcome the limitations imposed by traditional data-dependent acquisition (DDA)-MS and targeted MS approaches for high-throughput, accurate proteome quantification. However, there was no CHO SWATH-MS spectral library publicly available; the potential commercial value and competing interest might have deterred the release of in-house spectral libraries generated by biopharmaceutical companies and contract research organizations. We understand that a comprehensive SWATH spectral library, which contains the empirically accurate SWATH assay coordinates, is imperative to extract high-quality quantitative measurements from the SWATH-MS data sets. Hence, with the aim to facilitate and improve the consistency in bioprocess development as well as to benefit the academic research, we believe the construction of a publicly accessible CHO-specific mass spectral library is of utmost importance to the future applications of SWATH-MS in biopharma R&D and biomanufacturing.
We are thrilled to share our findings from the in-depth investigation of CHO cell proteome with the scientific community. In our publication in Scientific Data, it presents the first comprehensive CHO SWATH-MS spectral library generated through a series of systematic analyses of mAb-producing CHO-K1 intracellular proteome using the advanced LC-MS technique coupled with multi-dimensional fractionation strategies. The paper details the methodology for the library generation and demonstrates the successful application of SWATH-MS in the quantification of CHO cell proteome obtained from different sources, including the cell lysates, harvested cell culture fluid and downstream mAb process intermediates. Our results have also demonstrated the feasibility of SWATH-MS with the in-house CHO SWATH spectral library as a potential process analytical technology (PAT) to resolve the bioprocessing issues within the quality by design (QbD) paradigm for future biopharmaceuticals manufacturing system.
With implementation of CHO HCPs reference and quantification standard for inter-labs instrument performance qualification and method validation across the world, we hope that the universal SWATH-MS method for host cell impurity analysis can be accepted and widely adopted by the regulatory authority and biopharmaceutical industry as alternative technique to overcome the current ELISA limitations: time consuming to develop satisfactory coverage, product & process specific Immunol assay, and unable to detect individual host cell proteins of risk for the drug safety and efficacy. Similar approach and workflow can be applied to characterize different host cell impurities and quality contributing active ingredients in different therapeutic protein product and processes, non-protein therapeutic modalities such as stem cells, viral vectors and vaccines.
With the advantage of robustness, reproducible and streamlined procedures, CHO spectral library aided SWATH-MS can be applied to different LC and MS setups in all DIA-MS capable laboratories from biopharma company R&D and biomanufacturing, CRO/CDMO to academic research. We are expecting that SWATH-MS with comprehensive CHO spectra library can be used as a routine process analytical technology (PAT) tool to better monitor the critical process parameters (CPPs) and/or CQA associated predictors, and return the feedback to define a design space in controlled consistent bioprocesses for CQA improvement enabling quality by design (QbD) in future bio-manufacturing.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in